ANSWER
The mass of water is 0.0536 kg
STEP-BY-STEP EXPLANATION;
Given information
The mass of KNO3 = 175g
The molarity of the solution = 32.25 M
The molality formula is given below as
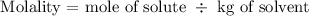
The first step is to find the mole of the solute using the below formula
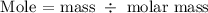
Recall, the molar mass of KNO3 is 101.1032 g/mol
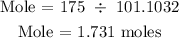
The second step is to find the mass of water using the molality formula
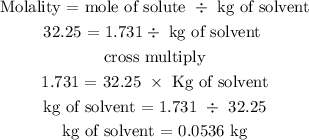
Hence, the mass of water is 0.0536 kg