If one substance is more polar than another, it will be more resistant to gravity. The mutual attraction of water molecules is called cohesion. This cohesion pulls a drop into a spherical shape, because that’s the shape that allow for the maximum number of bonds.
For the oil we have:
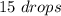
For the alcohol we have:
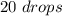
So:
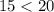
We can conclude oil is less polar than alcohol, it can’t resist gravity, and all of the molecules are pulled down into a flat sheet over the penny’s surface. This flat sheet can accommodate many fewer drops of oil than the hydrogen-bond induced sphere that’s on the penny with alcohol.