Answer
Step-by-step explanation
Given:
Volume of chlorine gas, V = 25.7 L,
Pressure, P = 1 atm, and
Temperature, T = 25 °C (25 + 273.15 = 295.15 K)
What to find:
The grams of carbon disulfide are needed to completely consume 25.7 L of chlorine gas.
Step-by-step solution
The question combines using (a) the ideal gas law, and (b) a stoichiometry calculation.
The first step is to write the balanced chemical equation for reaction as shown below:
CS₂ (s) + 4Cl₂ (g) --> CCl₄ (l) + 2SCl₂ (s)
The second step is using the ideal gas law to calculate the number of moles of Cl₂ gas as follows:
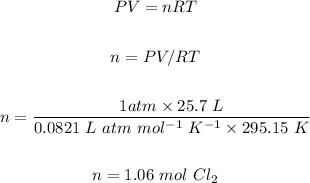
The next step is using the balanced chemical reaction and stoichiometry to convert moles of Cl₂ to moles of CS₂.
From the reaction, you can see that 4 moles of Cl₂ react with 1 mole of CS₂. Therefore, 1.06 moles Cl₂ will react with:
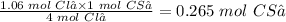
The final step is to convert 0.265 mol CS₂ to grams CS₂ using its molar mass (76.139 g/mol).
1 mol CS₂ = 76.139 grams CS₂
Therefore 0.265 mol CS₂ will be equal
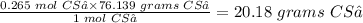
The answer is: 20.18 grams carbon disulfide