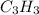Answer:
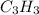
Step-by-step explanation:
Here, we want to get the molecular mass of the compound given its empirical formula
The difference between the two is simply the subscript attached to the atoms which is the number of these atoms
Let us call the number n
We should recall that the atomic mass unit of carbon is 12 amu , while the atomic mass unit of hydrogen is 1 amu
In its final outlook, the molecular formula would be:
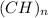
We can use the individual amu to get the value of n
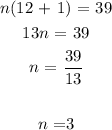
Thus means the molecular formula of the compound is: