Answer
a) NaI
b) NaI
c) HOCl
Step-by-step explanation:
Given reaction:
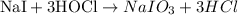
a) The substance oxidized:
The substance oxidized is NaI because the oxidation number of iodine in NaI goes from -1 to +5 in NaIO₃
b) The species that functions as the reducing agent:
The species functions as the reducing agent is NaI. This is because the oxidizing agent is the substance reduced and the reducing agent is the substance oxidized
c) The substance reduced:
The substance reduced is HOCl because the oxidation number of chlorine in HOCl goes from +1 to -1 in HCl