To find the limiting reactant we have to divide each amount of moles by the stoichiometric coefficient of the corresponding substance.
It means that we have to divide the number of moles of C3H8 by 1 and the number of moles of O2 by 5.
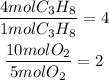
The least quotient will indicate that that substance is the limiting reactant.
It means that the limiting reactant is O2.