1) Balance the chemical equation.
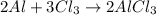
2) List the known and unknown quantities.
Reactant: Cl2
Mass: 16.4 g
Product: AlCl3
Mass: unknown (g).
3) Convert the mass of Cl2 to moles of Cl2.
The molar mass of Cl2 is 70.9060 g/mol.
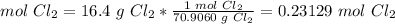
4) Convert moles of Cl2 to moles of AlCl3.
The molar ratio between Cl2 and AlCl3 is 2 mol Cl2: 2 mol AlCl2.
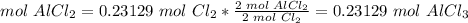
5) Convert moles of AlCl3 to mass of AlCl3.
The molar mass of AlCl3 is 133.3405 g/mol.
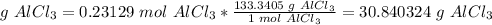
The theoretical yield rounded to the nearest gram is 31 g AlCl3.
Option A: 32