Answer
The grams of cholesterol in 610 mL of blood to one significant figure = 1.0 g
Step-by-step explanation
Given:
The concentration of cholesterol (C27H46O) in normal blood = 0.005 M
The volume of blood = 610 mL = 0.610 L
What to find:
The grams of cholesterol in 610 mL of blood.
Step-by-step solution:
Step 1: Determine the moles of cholesterol.
The number of moles of cholesterol in the blood can be calculated using the molarity formula, which is
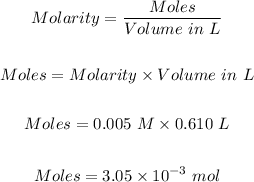
Step 2: Calculate the molar mass of cholesterol.
Using the atomic masses of (H = 1.00784, C = 12.011, O = 15.999) from the periodic table, the molar mass of cholesterol is calculated as follows:
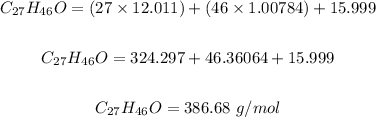
Step 3: Determine the grams of Cholesterol.
The grams of cholesterol in 610 mL of blood will be:
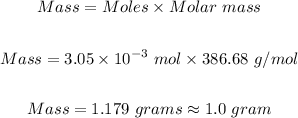
Hence, the grams of cholesterol in 610 mL of blood to one significant figure is 1.0 g.