Explanation:
Firstly, we need to write out the balanced equation of the reaction
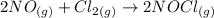
Given parameters
• The equilibrium constant of the reaction = 52
,
• The partial pressure of NO at equilibrium = 0.240 atm
,
• The partial pressure of Cl at equilibrium = 0.608 atm
According to the balanced equation, 2 moles of NO reacts with 1 mole of Cl to give 2 moles of NOCL
The equilibrium constant of the reaction is given below
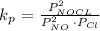
Substituting the parameters into the formula above
![\begin{gathered} 52\text{ = }\frac{P^2_(NOCL)}{(0.240)^2\cdot\text{ 0.608}} \\ 52\text{ = }\frac{P^2_(NOCL)}{0.0576\cdot\text{ 0.608}} \\ 52\text{ = }(P^2_(NOCL))/(0.0350) \\ \text{Cross multiply} \\ 52\cdot0.0350=P^2_(NOCL) \\ 1.821=P^2_(NOCL) \\ \text{Take the square roots of both sides} \\ \sqrt[]{1.821}\text{ = }\sqrt[]{P^2_(NOCL)} \\ P_{NOCL\text{ }}=\text{ }1.350\text{ atm} \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/u565yckraz6id3jkpi5snh8gyeik10mdwu.png)
Hence, the partial pressure of NOCL at 500K is 1.350atm