Answer:
250 g of hydrogen (H2).
Step-by-step explanation:
What is given?
Molecules of oxygen (O2) = 3.77 x 10²⁵ molecules.
Molar mass of hydrogen (H2) = 2 g/mol.
Step-by-step solution:
Let's state the chemical equation where hydrogen is H2, oxygen is O2, and water is H2O:
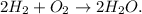
First, we have to use the Avogadro's number, which is 6.022 x 10²³ molecules/mol. This number is telling us that there are 6.022 x 10²³ molecules in 1 mol of a substance. This is because we have to do the conversion from molecules to moles of O2:
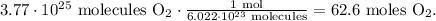
Now that we have 62.6 moles of O2, we have to find what is the number of moles of hydrogen (H2) required to react with this amount. You can see in the chemical equation that 2 moles of H2 react with 1 mol of O2:
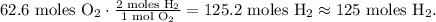
And the final step is to convert from 125 moles of H2 to grams using its molar mass:
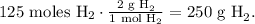
The answer is that we require 250 g of hydrogen (H2) to react with 3.77 x 10²⁵ molecules of oxygen (O2).