Answer: 92.50%
From the given problem, we can say that the total volume of the concentration is:
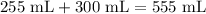
The initial volume of the solution is 555 mL. After the evaporation, the 50% sucrose solution lost 255 mL.
To find the concentration of the remaining 300 mL, we will use the following formula:
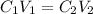
Where:
C₁ = initial concentration
C₂ = final concentration
V₁ = initial volume
V₂ = final volume
From the given, we know that:
C₁ = 50% = 0.50
C₂ = ?
V₁ = 555 mL
V₂ = 300 mL
Substitute these to the formula and we will get:
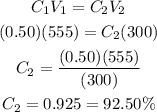
Therefore, we can say that the sucrose concentration in the remaining 300mL is 92.50%