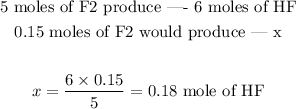Step 1 - Finding the stoichiometry of the reaction
The given reaction is:
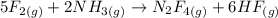
The stoichiometry of the reaction can be found by the bigger numbers that come before the formula of the substance:
5 moles of F2 react with 2 moles of NH3 thus producing 1 mole of N2F4 and 6 moles of HF
Since the exercise is specifically asking about the relation between F2 and HF, we can simplify this statement to:
5 moles of F2 produce 6 moles of HF
Note that this is a fixed relation, and we'll be using it to solve the question.
Step 2 - Using the stoichiometry to discover how many moles of HF would be produced
We can now set the following proportion, since we already know the stoichiometry: