To answer this question, we will need to use an ICE table.
So, we start with the concentration we want to calculate, [HClO].
The dissociation equilibrium is:
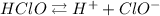
At start, we only have HClO, so the first row of the table is:
HClO | H⁺ | ClO⁻
[HClO]i | 0 | 0
Now, suppose x dissociates at the equilibrium, so we would have:
HClO | H⁺ | ClO⁻
[HClO]i | 0 | 0
[HClO]i - x | x | x
Since the concentration of H⁺ will be x, we can calculate it by using the given pH:
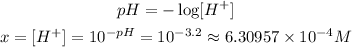
Now, we can figure the initial concentration of HClO by using the equilibrium equation:
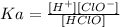
From the ICE table, we have:
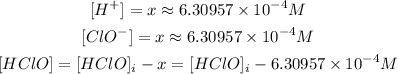
Thus:
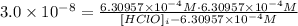
Now, we can solve for [HClO]i:
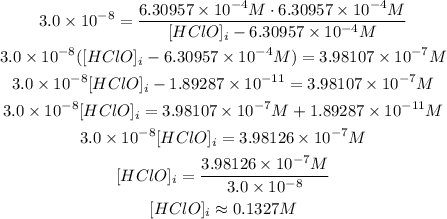
Thus, the initial concentration is approximately 0.1327 M.