Answer:
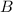
Step-by-step explanation:
Here, we want to write the formula for the weak base ionization constant for HCO3
Firstly, we write the ionization equation as follows:
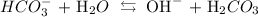
In writing the constant, we do not add the concentration of water
We have the equation as:
![([H_2CO_3][OH^-])/([HCO_3^-])](https://img.qammunity.org/2023/formulas/chemistry/college/5ju8rwtktasbwv0gu8fh84eew2gugepww5.png)
Thus, the second option is the correct answer choice