We must first find the moles corresponding to 81.7 grams of ethylene. To do this, we divide the grams by the molar mass of ethylene: 28.05g/mol.
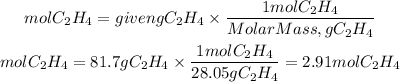
Now, by stoichiometry, we see that the ratio of acetaldehyde to ethylene is 2/2. So, the moles of C2H4O will be:
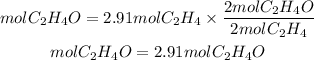
Now, to find the grams of C2H4O we will need the molar mass of C2H4O. The molar mass of C2H4O is 44.05g/mol. The grams of C2H4O will be:
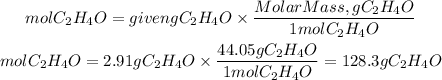
Answer: The grams of acetaldehyde that can be prepared are 128.3 grams