Given data:
Mass of water:
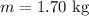
Initial temperature of water:
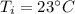
Energy required to boil water is given as,
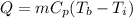
Here, Cp is the specific heat capactity of water, and Tb is the boiling temperature of water.
Substituting all known values,
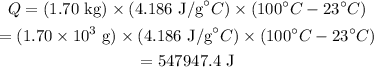
Converting Joules to cal;
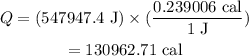
Therefore, 130962.71 cal of energy required to completely boil 1.70 kg of water at 23°C.