What remains constant before and after the addition of water is the number of moles in the beaker.
So, let the concentration be c₁ and c₂ (before and after), the number of moles be n and the volume before be V₁ and after be V₂.
The equation for concentration is number of moles divided by the volume, so we have, for before the addition:
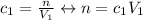
And the equation for after:
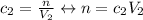
Notice that we didn't put subscripted number on n because this won't change.
Because n doesn't change, we can say:
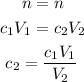
We know c₁ is 0.14 mol/L and V₁ is 150 mL.
The volume after the addition will be the volume before plus the amount we added, so:
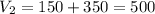
Thus, the final concentration will be:
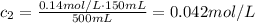
The final concentration will be 0.042 mol/L.