When calculating concentration, we always need to remember that the number of moles remain constant.
The formula for concentration is:
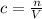
So, if we don't add or remove solute, n will remain constant, while c and V vary.
If we want a solution of chloride ion concentration of 0.100 mol/L and 250 mL of volume, we need to first convert 250 mL to L:
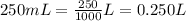
Now, we can use the formula for concentration to calculate the number of moles:
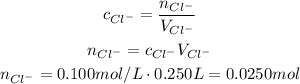
Now, the dissociation of MgCl₂ will be:
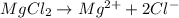
So, for each mol of MgCl₂, we will have 2 moles of Cl⁻.
So, if we need 0.0250 mol of Cl⁻, we need half as many of MgCl₂, that is:
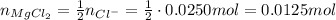
Now, we need to calculate the volume necessary of the solution of 0.500 mol/L MgCl₂:
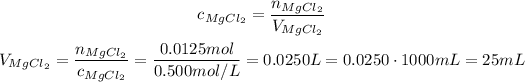
So, we will need 25 mL os the solution.