Answer:
1.01 * 10^7 grams/day
Explanations:
Given the reaction produced during the combustion of methane expressed as:
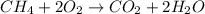
Calculate the moles of methane:
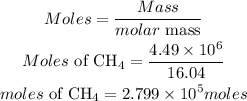
According to stochiometry, 1 mole of methane produces 2 moles of water. The moles of water produced at the end of the reaction will be:
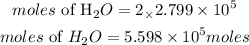
Determine the mass of water produced in a day
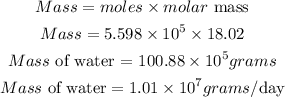
Hence the mass in grams of water produced by this reaction in one day is approximately 1.01 * 10^7 grams