To solve this question we must use the ideal gas law:

Where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant and T is the temperature (in kelvin degrees).
Solve the equation for T and replace for the given values. Remember that R has a value of 0.082atmL/molK:
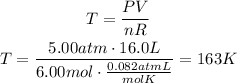
The temperature inside the tank is 163K.