We will find the average change as follows:
*First, we determine the points, those are:
(2015, x)
(2019, x - 168)
Now, the average change is determined as follows:
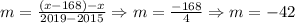
So, the average change is -42 students per year.
***Explaination***
Since we are given two dates and two quantities we can interpret them as points.
We don't know the total number of students but we know the change over time, so we assign a random value to represent the total number of students, it will be "x" and it will be at "its maximum" in 2015.
So, we have our first point (2015, x)
Now, we know that the total number of students changed by -168 from 2015 to 2019, so in 2019 we will have "x - 168" students.
So, we have our second point (2019, x - 168).
*We know that the average change is given by the change on y divided by the change on x [The slope of both points], and since we have that the points follow (x1, y1) & (x2, y2), we will replace in the following expression [Slope]:
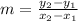
Since we know the following:
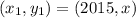
&
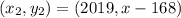
We replace in the expression:
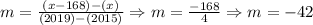
So, the average change was -42 students per year.