Answer:
The molarity of the solution is 0.697M.
Step-by-step explanation:
Molarity represents the moles of solute that are contained in 1000mL (1L) of solution.
In this case, we have to calculate the molarity of 703mL solution that contain 0.490 moles:
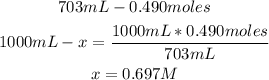
So, the molarity of the solution is 0.697M.