Answer:
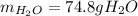
Step-by-step explanation:
Hello there!
In this case, according to the given chemical reaction, it is possible to realize there is a 1:2 mole ratio of sulfuric acid to water; thus, given the mass of the former and its molar mass (98.07 g/mol), it is possible to determine the mass of produced water as shown below:
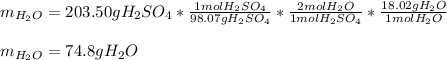
Regards!