Matter and Change - Mixture
A mixture is a combination of two or more substances in any proportion that do not combine chemically. In this case urea, propylen glycol and crème base.
Answer:
First we calculate the mass of the formulation as it is origynally prepared:
For this we calculate the mass of propylene glycol using its density (1.04 g/ml):
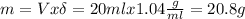
Now we calculate the total mass:
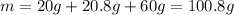
Now we know that 20g of urea are used to prepare 100.8g of the formulation, so we calculate the mass for 18g of the formulation:
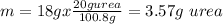
So the answer is 3.57g of urea.