Answer:
![C_3H_8+5O_2\operatorname{\rightarrow}3CO_2+4H_2O.]()
Step-by-step explanation:
Let's see the unbalanced chemical equation:
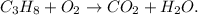
You can note that all the species are unbalanced because on the reactant side we have 3 carbons, 8 hydrogens, and 2 oxygens, whereas on the product side we have 1 carbon, 2 hydrogens, and 3 oxygens.
If we start balancing carbon by putting '3' moles beside CO2, we will have 3 carbons for both sides:
![C_3H_8+O_2\operatorname{\rightarrow}3CO_2+H_2O.]()
And we can balance hydrogen by putting '4' moles beside H2O to obtain 8 hydrogens for both sides:
![C_3H_8+O_2\operatorname{\rightarrow}3CO_2+4H_2O.]()
Now, you can note that on the product side we have 10 oxygens so if we put '5' moles beside O2 we will have 10 oxygens for both sides and we will have the balanced chemical equation done:
![C_3H_8+5O_2\operatorname{\rightarrow}3CO_2+4H_2O.]()