Answer:
3.575grams
Explanations:
From the question, we will need to determine the moles of Na₂CO3. 10H₂O using the formula below;
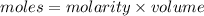
Given the following parameters
Molarity = 0.05M
Volume = 250ml = 0.25L
Determine the moles of Na₂CO3. 10H₂O
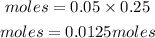
Determine the Na₂CO3. 10H₂O
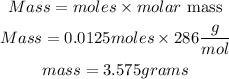
Hence the mass of Na₂CO3. 10H₂O required to prepare a 250 ml of a 0.05 M solution is 3.575grams