Answer:
3.53moles
Explanations
The formula for calculatung the moles of the compound is expressed as:
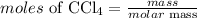
Given the following
Mass of CCl4 = 543.2 grams
Determine the molar mass of CCl4
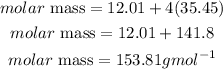
Determine the required mole
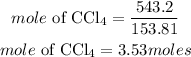
Hence the moles of tetrachloride is 3.53moles