To find the unit cost, we need to find the cost per ounce of tomato sauce.
The total cost was: $3.29
And the total number of ounces was: 28 oz
To find the unit cost or the cost per ounce we divide the total cost by the total number of ounces:
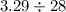
The result of this division is:
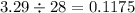
which can be round to 0.12, which is option a.
Answer:
a. $.12