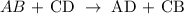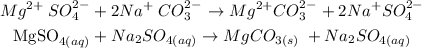Explanation:
Given information
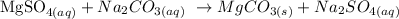
The above reaction is a double displacement reaction. In this type of reaction, ions get exchanged between two reactants which form new compounds called the products.
The general chart for this reaction is written below as