Answer:
C₅H₇N
Explanations:
To determine the empirical formula of nicotine, we will follow the following steps:
Determine the moles of each element
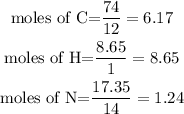
Divide through by the smallest ratio.
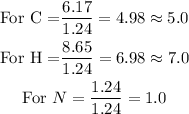
Determine the empirical formula.
The ratios show that there are 5 atoms of Carbon, 7 atoms of hydrogen, and 1 mole of Nitrogen in the empirical formula of nicotine. Hence the empirical formula of nicotine will be C₅H₇N