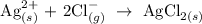ANSWER
Step-by-step explanation
Given that;
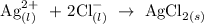
The equation above represents a direct combination of elements to give compound.
The subscripts attached to each element is incorrect because silver is a solid metal and it should exist in solid state.
Chlorine is a diatomic gas and the state which it should exist should be the gaseous state.
Hence, the new equation is