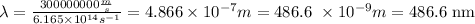A photon of light with frequency of 6.165 x 10^14 Hz has a wavelength of 486.6 nm and energy of 4.084x10^-19 J
The energy of a photon is defined as
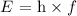
where h is the Planck's constant and f is the frequency.
h is a constant of value h = 6.62607004 × 10^-34 m2 kg / s
and f was given in the question, f = 6.165 x 10^14 Hz (keep in mind that 1 Hz = 1/s)
Then we calculate:
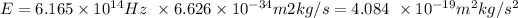
Since the unit Joules corresponds to (m^2 kg / s^2), we have that E = 4.084x10^-19 J, and we may calculate the wavelength of the photon considering that:
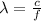
where λ is the wavelength, c is the velocity of light and f is the frequency.