We know that there are 3.52 moles of krypton and we must find how many atoms there are
In oder to find the number of atoms we must use the next formula
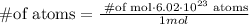
We need replace in the formula the given information,
- # of mol = 3.52
Now, replacing in the formula
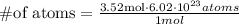
Simplifying,
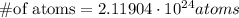
ANSWER:
In 3.52 moles of krypton there are 2.11904 * 10^24 atoms.