The question requires us to calculate the mass of Cu(NO3)2 obtained when 7.20 moles of AgNO3 are used with the following chemical reaction:
Cu + 2 AgNO3 → Cu(NO3)2 + 2 Ag
To solve this question, we'll need to go through the following steps:
1) Confirm if the chemical equation provided is balanced;
2) Use the stoichiometric relation from the reaction to calculate the amount of Cu(NO3)2, in moles, that would be obtained;
3) Calculate the molar mass of Cu(NO3)2;
4) Use the molar mass obtained in step 3 and the number of moles obtained in step 2 to calculate the mass of Cu(NO3)2 that would be produced.
Next, we'll go through these steps to solve the question:
1) Looking at the chemical reaction provided, we can see that there are the same amount of atoms for all elements on both sides of the equation, thus the reaction is already balanced and we don't need to change the coefficients.
2) Next, we need the stoichiometric relation between AgNO3 and Cu(NO3)2, given by the reaction, to calculate the number of moles of Cu(NO3)2 that would be produced from 7.20 moles of AgNO3.
From the reaction, we can see that 2 moles of AgNO3 are required to obtain 1 mol of Cu(NO3)2. Then, we can write:
2 mol AgNO3 -------------------------- 1 mol Cu(NO3)2
7.20 mol AgNO3 --------------------- x
Solving for x, we have:
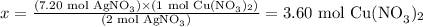
Therefore, 3.6 mol of Cu(NO3)2 are produced from 7.2 mol of AgNO3.
3) Now, we need the molar mass of Cu(NO3)2, which we can calculate from the atomic masses of Cu, N and O and the amount of atoms of these elements in the molecule.
The atomic masses of Cu, N and O are 63.55, 14.01 and 15.99 u, respectively.
molar mass Cu(NO3)2 = (1 * 63.55) + (2 * 14.01) + (6 * 15.99) = 187.51 g/mol
4) At last, we use the molar mass calculated (187.51 g/mol) and the number of moles obtained (3.6 moles) to calculate the mass of Cu(NO3)2 that would be produced:
1 mol Cu(NO3)2 --------------------- 187.51 g Cu(NO3)2
3.6 mol Cu(NO3)2 ----------------- x
Solving for x, we have:
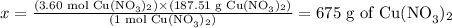
Therefore, according to the reaction provided, 7.20 moles of AgNO3 would yield to 675 g of Cu(NO3)2.