ANSWER
The molarity of the solution is 0.014
Step-by-step explanation
Given that;
The mass of the dissolved KCl is 0.62g
The volume of water is 600ml
Follow the steps below to find the molarity of the solution
Step 1; Find the molar mass of the solute

Therefore, the molar mass of KCl is 74.553 g/mol
Step 2; Convert the volume of the solution to L
Recall, that 1 mL is equivalent to 0.001L
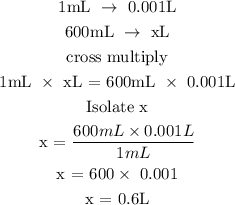
Step 3; Find the number of moles of KCl using the below formula
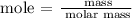
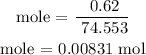
Step 4; Find the molarity of the solution using the below formula
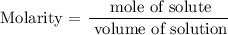
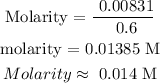
Therefore, the molarity of the solution is 0.014