The first step to answer this question is to convert the given mass of Na to moles:
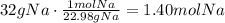
Using the stoichiometric ratio, we know that 2 moles of Na produce 2 moles of NaCl:
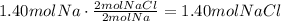
Use the molecular weight of NaCl to convert the number of moles to grams:
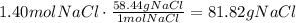
Now, divide the actual yield of NaCl by the theoretical yield of NaCl and multiply it by 100:
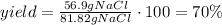
It means that the correct answer is c. 70%.