ANSWER
Step-by-step explanation
Single replacement reaction is a type of reaction in which one element is substituted for another element in a compound.
Hence, we have the below reaction as one of the the examples of a single replacement reaction
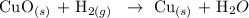
In the above reaction, the hydrogen atom displaced the copper atom in CuO. This is because hydrogen is more reactive than copper in the activity series chart.