We will convert the mass of reactants to moles:
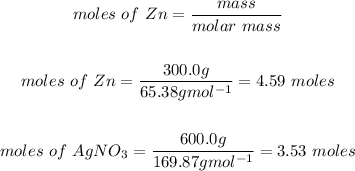
We will now determine the limiting reactant by using the mole to coefficient ratio. The lowest ratio is the limiting reactant:
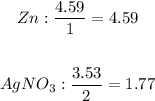
-The silver nitrate has the lowest ratio and is therefore the limiting reactant. The limiting reactant determines how much product can be formed.
We will determine the mass of silver that can be formed from this 600 grams of silver nitrate:
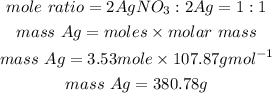
Answer: Based on the calculations we see C) No, the silver nitrate will run out first.