Answer: mass of Oxygen required = 17.45 g
Calculations:
• Molar mass NH3 ,= Molmass of N + ( 3xmol.mass of H ) = ,17 g/mol
,
• Molar mass of O2, = 2 x Mol.mass of O = 2*16 = ,32 g/mol
,
• Mass of NH3 = 7.42 g
The balanced equation of the chemical reaction:
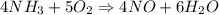
According to stoichemistry ,
• 4 moles of NH3 reacts with 5 moles of O2
,
• 4x17 g of NH3 reacts with 5x32 g of O2
• then , 7.42 g NH3 reacts with x mass of O2
Therefore mass of Oxygen will be :
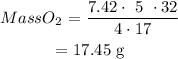
This means that mass of Oxygen required = 17.45 g