Answer: Option B
Explanation :
An arrhenius acid is an acid that dissociates in water to form hydrogen ions (H + ).
Example :
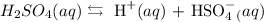
Dissociation of an arrhenius acid that yields H+ ion .
{extra notes , on thearrhenius base dissociate in water to form OH- ions }