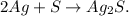This would be a combination reaction or synthesis reaction. A combination reaction is a reaction in which two or more substances combine to form a single new substance. The general form of a combination reaction is:
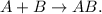
Silver metal is represented by Ag and sulfur powder is represented by S. The combination of these two elements would be Ag2S, because the oxidation state of silver (Ag) is +1, whereas the oxidation state of sulfur is -2, so the algebraic sum between these two elements must be zero. This means that we need two silvers to bond with an atom of sulfur because sulfur can bond twice. The chemical equation could see like this:
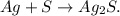
We need to balance Ag but it's very simple, we just have to put '2' moles beside Ag in the reactant and we're going to obtain the balanced chemical equation: