In order to calculate the amount of heat (in Joules) required to heat the water, we can use the formula below:
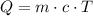
Where Q is the energy, m is the mass, c is the specific heat and T is the change in temperature.
Since the given specific heat is in grams (J/g°C), we need to use the mass in grams as well.
(we can also change the specific heat to kg and the mass to kg, but the result is the same)
So we have:
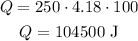
If we change the mass to kg (m = 0.25 kg) and the specific heat to kg (c = 4180 J/kg°C), the equation is:
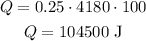
Therefore the correct option is D.