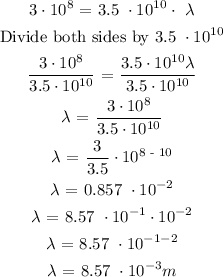ANSWER
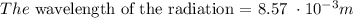
Explanation:
What to find? The wavelength of the electromagnetic radiation.
Given parameters
Frequency of the electromagnetic radiation = 3.5 * 10^10Hz
To find the wavelength of the electromagnetic radiation, we need to apply the below formula

Note: The speed of light is given as 3 x 10^8 m/s
The next thing to do is to substitute the given parameters into the above formula