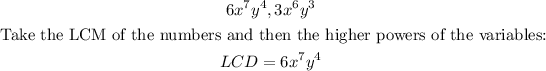The given fractions are:
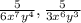
It is required to find the least common denominator (LCD).
Recall that the least common denominator (LCD) is the smallest term that can be a common denominator for a set of fractions.
To find the LCD, find the least common multiple of the expressions in the denominators: