Answer
1.50 mol
Step-by-step explanation
Given:
Initial volume, V₁ = 25.0 L
Mass of CH4 gas in 25.0 L container = 40 g
Final volume, V₂ = 15.0 L
From the Periodic Table; molar mass of CH4 = 16.04 g/mol
What to find:
The new amount in mole.
Step-by-step solution:
According to Avogadro’s law: For a confined gas, the volume (V) and number of moles (n) are directly proportional if the pressure and temperature both remain constant. That is:
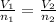
n₁ = Mass/Molar mass = (40.0g/16.04 g/mol) = 2.493765586 mol
n₂ is the new amount in mole and can be calculated as follows:
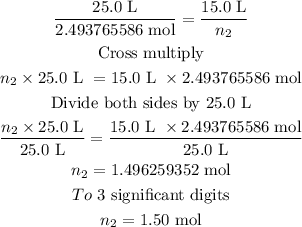
The new amount in moles is 1.50 moles