1) Write the chemical equation.
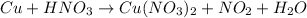
Step 1: assign letters to stoichiometric coefficients.
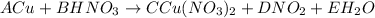
Step 2: list the elements involved in the reaction.
Cu: 1A = 1C
H: 1B = 2E
N: 1B = 2C + 1D
O: 3B = 6C + 2D + 1E
Step 3: assign an arbitrary number to one of the letters. Let's try C = 1.
Cu: 1A = 1C
1*A = 1*(1)
A = 1
Let's try E = 2.
H: 1B = 2E
1*B = 2*(2)
B = 4
Replace C and B in 1B = 2C + 1D
N: 1B = 2C + 1D
1*(4) = 2*(1) + 1D
4 = 2+ 1*D
D = 4-2 = 2
Step 4: replace the letter in the chemical equation and check.
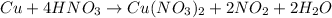
.