The balanced equation is:
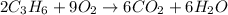
To balance these equations, always start with Carbon, followed by Hydrogen and then do Oxygen last.
First thing to do is to write the atoms on each side of the equation.
Left of equation Right of the equation
C = 3 C = 1
H = 6 H = 2
O = 2 O = 3
Next thing to do is to balance the carbons first.
On the left we have 3 and on the right we have 1
so 1 x 3 to balance the carbons
C3H6 + O2 = 3CO2 + H2O
for hydrogen we to get 6, we need to say 2 x 3
C3H6 + O2 = 3CO2 + 3H2O
Now if we put 3 infront of H2O on the right, There will be 3 more Oxygens added as well, and since we put 3 infront of CO2 to balance the Carbons, there are 3 more Oxygens added again. Now the number of oxygens on the left is not 3 but it is a 9.
To balance the 9 oxygens on the right with 2 oxygens on the left we have to multiply the oxygens on the left by 9/2
C3H6 + (9/2) O2 = 3CO2 + 3H2O
So we do not want a fraction on our balanced equation, the next thing to do is to multiply everything by 2, to get rid of the fraction.
2 x [C3H6 + (9/2) O2 = 3CO2 + 3H2O the 2 will cancel the 2 from the fraction.
After multiplying everything by 2 we get:
2C3H6 + 9O2 = 6CO2 + 6H2O
Which is your final answer.