Answer: The enthalpy change for formation of butane is -125 kJ/mol
Step-by-step explanation:
The balanced chemical reaction is,
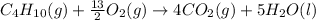
The expression for enthalpy change is,
![\Delta H=[n* H_f{products}]-[n* H_f{reactants}]](https://img.qammunity.org/qa-images/2022/formulas/chemistry/high-school/vjsuja8qv86fpr4xps3gs5.png)
Putting the values we get :
![\Delta H=[4* H_f_(CO_2)+5* H_f_(H_2O)]-[1* H_f_{C_4H_(10)}+(13)/(2)* H_f_(O_2)]](https://img.qammunity.org/qa-images/2022/formulas/chemistry/high-school/wrh9vbc1qjxggf49fw5y6m.png)
![-2877=[(4* -393)+(5* -286)]-[1* H_f_{C_4H_(10)}+(13)/(2)* 0]](https://img.qammunity.org/qa-images/2022/formulas/chemistry/high-school/hryd3rv6wzzg5cpflpw75k.png)
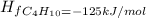
Thus enthalpy change for formation of butane is -125 kJ/mol