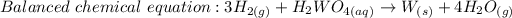
We will convert the mass of tungsten into moles:
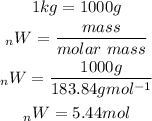
Based on the chemical equation 3 moles of hydrogen produces 1 mole tungsten. We will use this information to determine the moles of hydrogen needed to produce 5.44 moles of tungsten:
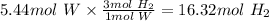
Answer: 16.32mol H2 is needed to prepare 1kg tungsten.