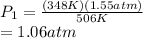Answer:
1.06 atm
Step-by-step explanation:
We are given the following variables to work with:
Initial pressure (P1): unknown
Initial temperature (T1): 348 K
Final temperature (T2): 506 K
Final pressure (P2): 1.55 atm
We are asked to find the initial pressure (P1). We want to choose a gas law equation that relates initial pressure and temperature to final pressure and temperature. Gay-Lussac's law does this:
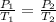
We can rearrange the law algebraically to solve for
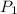 .
.
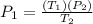
Substitute your known variables and solve: